
Chemistry, 09.06.2020 19:57 josephicarusmarrujo
At high temperatures one mole of hydrogen gas reacts with one mole of bromine gas to form hydrogen bromide. At a given temperature the equilibrium constant is 57.6. If at the same temperature, a mixture of 4.67 × 10^-3M bromine gas, 2.14 × 10^−3 hydrogen gas, and 2.40 × 10^−2M hydrogen bromide gas is made, then:
a. the system is at equilibrium.
b. the system is far from equilibrium and will shift to form more hydrogen gas.
c. the system is far from equilibrium and will shift to form more hydrogen bromide gas.
d. nothing can be deduced since we do not know whether the reaction is endothermic or exothermic.
e. nothing can be deduced since we do not know whether the equilibrium constant is Kc or Kp.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2
You know the right answer?
At high temperatures one mole of hydrogen gas reacts with one mole of bromine gas to form hydrogen b...
Questions

Advanced Placement (AP), 16.01.2021 01:40


History, 16.01.2021 01:40


Mathematics, 16.01.2021 01:40


Biology, 16.01.2021 01:40




Social Studies, 16.01.2021 01:40

Advanced Placement (AP), 16.01.2021 01:40

Mathematics, 16.01.2021 01:40



Physics, 16.01.2021 01:40




Mathematics, 16.01.2021 01:40

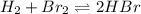
![Keq=\frac{[HBr]^2}{[H_2][Br_2]} =57.6](/tpl/images/0680/9707/6b896.png)
![Q=\frac{[HBr]^2}{[H_2][Br_2]} =\frac{(2.40x10^{-2})^2}{(4.67x10^{-3})(2.14x10^{-3})} \\\\Q=57.6](/tpl/images/0680/9707/53e8b.png)


