
Chemistry, 08.06.2020 23:57 raylynnreece4939
A second - order reaction has a rate constant of 0.06 M min - 1 . It takes min for the reactant concentration to decrease from 0.13 M to 0.088 M. Select one : O a . 73.4 O b . 7.80 O c . 6.50 O d . 61.2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
A second - order reaction has a rate constant of 0.06 M min - 1 . It takes min for the reactant con...
Questions


Mathematics, 18.09.2019 03:00

Mathematics, 18.09.2019 03:00

Biology, 18.09.2019 03:00

Mathematics, 18.09.2019 03:00









Physics, 18.09.2019 03:00

Spanish, 18.09.2019 03:00



Arts, 18.09.2019 03:00

History, 18.09.2019 03:00

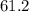 minutes.
minutes.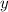 denote the concentration of that reactant (in
denote the concentration of that reactant (in 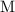 .)Let
.)Let 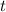 denote time (in minutes.)
denote time (in minutes.)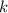 denote the rate constant of this reaction. Assume that
denote the rate constant of this reaction. Assume that 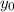 is the concentration of that reactant at the beginning of this reaction (when
is the concentration of that reactant at the beginning of this reaction (when 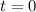 .) Because this reaction is of second order, it can be shown that:
.) Because this reaction is of second order, it can be shown that: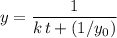 .
.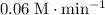 . Hence,
. Hence, 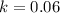 . For simplicity, assume that
. For simplicity, assume that 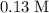 . Therefore,
. Therefore, 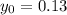 . The equation for concentration
. The equation for concentration 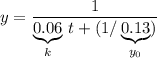 .
.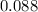 . That's the same as solving this equation for
. That's the same as solving this equation for 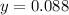 :
: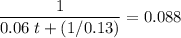 .
.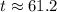 .
.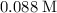 .
.

