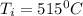Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
A 360. g iron rod is placed into 750.0 g of water at 22.5°C. The water temperature rises to 46.7°C....
Questions

Mathematics, 10.02.2021 07:30

Mathematics, 10.02.2021 07:30

Mathematics, 10.02.2021 07:30


Mathematics, 10.02.2021 07:30





History, 10.02.2021 07:30

Mathematics, 10.02.2021 07:30

Health, 10.02.2021 07:30


Mathematics, 10.02.2021 07:30

Mathematics, 10.02.2021 07:30


Computers and Technology, 10.02.2021 07:30



Mathematics, 10.02.2021 07:30

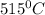
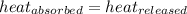
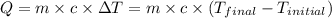
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0679/0203/09236.png) .................(1)
.................(1)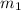 = mass of iron = 360 g
= mass of iron = 360 g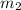 = mass of water = 750 g
= mass of water = 750 g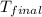 = final temperature =
= final temperature = 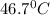
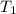 = temperature of iron = ?
= temperature of iron = ?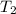 = temperature of water =
= temperature of water = 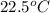
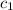 = specific heat of iron =
= specific heat of iron = 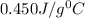
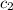 = specific heat of water=
= specific heat of water= 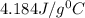
![-360\times 0.450\times (46.7-x)=[750\times 4.184\times (46.7-22.5)]](/tpl/images/0679/0203/039f8.png)