CaCO3(s)

Chemistry, 06.06.2020 08:58 CjTheStudentLOL
17. Consider the reaction shown and identify the statement that is not true.
825°C
CaCO3(s)
+ CaO(s) + CO2(g)
a.
This reaction is balanced as written.
b. The reactant must be heated for this reaction to occur.
c. The products are a solid and a gas.
d. Water must be present for this reaction to occur.
There are no solutions used in this reaction.
e.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

You know the right answer?
17. Consider the reaction shown and identify the statement that is not true.
825°C
CaCO3(s)
CaCO3(s)
Questions




Health, 07.07.2019 17:30

Mathematics, 07.07.2019 17:30

Mathematics, 07.07.2019 17:30

Biology, 07.07.2019 17:30

English, 07.07.2019 17:30

History, 07.07.2019 17:30



Physics, 07.07.2019 17:30

History, 07.07.2019 17:30

Mathematics, 07.07.2019 17:30


Social Studies, 07.07.2019 17:30

Advanced Placement (AP), 07.07.2019 17:30

Social Studies, 07.07.2019 17:30

Spanish, 07.07.2019 17:30

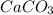 decomposition is:
decomposition is: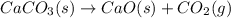
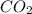 as product.
as product.

