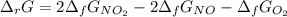(A) - 9.3 kcal

Chemistry, 06.06.2020 02:57 glowbaby123
Calculate the free energy change for the reaction:
2 NO(g) + O2(g) → 2 NO2(g)
(A) - 9.3 kcal
(B) + 24.9 kcal
(C) + 9.3 kcal
(D) - 16.6 kcal
(E) + 16.6 kcal


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

You know the right answer?
Calculate the free energy change for the reaction:
2 NO(g) + O2(g) → 2 NO2(g)
(A) - 9.3 kcal
(A) - 9.3 kcal
Questions


Chemistry, 26.04.2021 01:00

Mathematics, 26.04.2021 01:00

History, 26.04.2021 01:00

Computers and Technology, 26.04.2021 01:00


Mathematics, 26.04.2021 01:00

Mathematics, 26.04.2021 01:00


Physics, 26.04.2021 01:00


Mathematics, 26.04.2021 01:00

Mathematics, 26.04.2021 01:00


Mathematics, 26.04.2021 01:00

Mathematics, 26.04.2021 01:00