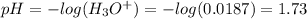A 1-liter solution contains 0.494 M hydrofluoric acid and 0.371 M potassium fluoride. Addition of 0.408 moles of hydrochloric acid will: (Assume that the volume does not change upon the addition of hydrochloric acid.)
a. Raise the pH slightly
b. Lower the pH slightly
c. Raise the pH by several units
d. Lower the pH by several units
e. Not change the pH
f. Exceed the buffer capacity

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
A 1-liter solution contains 0.494 M hydrofluoric acid and 0.371 M potassium fluoride. Addition of 0....
Questions

English, 27.08.2019 07:50

Mathematics, 27.08.2019 07:50


English, 27.08.2019 07:50


Mathematics, 27.08.2019 07:50

Computers and Technology, 27.08.2019 07:50

Mathematics, 27.08.2019 07:50



Mathematics, 27.08.2019 07:50




History, 27.08.2019 08:00




Spanish, 27.08.2019 08:00

Mathematics, 27.08.2019 08:00

![pH = pKa + log(\frac{[KF]}{[HF]})](/tpl/images/0677/3728/a79c6.png)

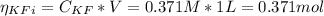
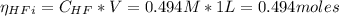

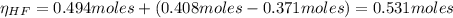
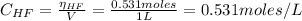
![Ka = \frac{[H_{3}O^{+}][F^{-}]}{[HF]}](/tpl/images/0677/3728/2de73.png)
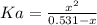 (2)
(2)