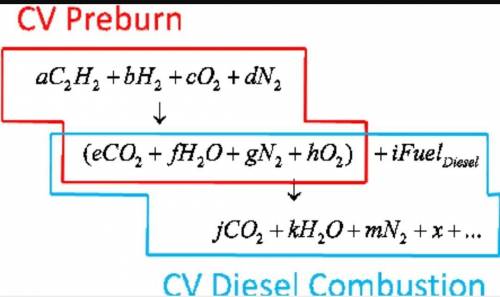
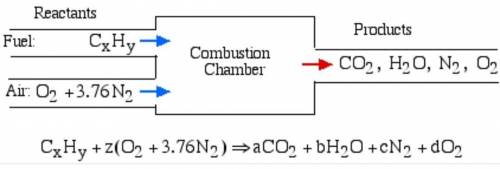
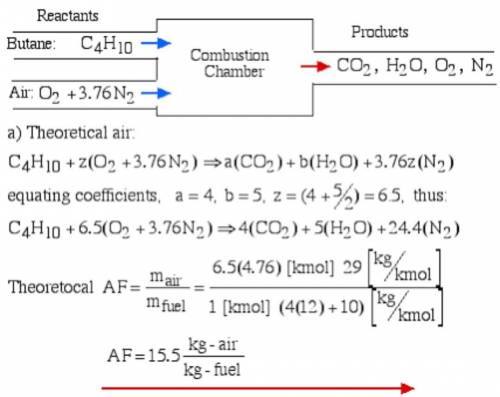
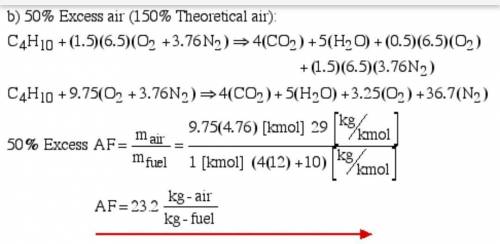
6.) A hydrocarbon molecule of
formula CxHy is completely
burnt in excess oxygen. The
comb...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Questions


Mathematics, 18.02.2021 05:20

Chemistry, 18.02.2021 05:20


Mathematics, 18.02.2021 05:20


World Languages, 18.02.2021 05:20

Mathematics, 18.02.2021 05:20






Mathematics, 18.02.2021 05:20

Biology, 18.02.2021 05:20



Mathematics, 18.02.2021 05:20