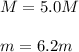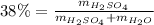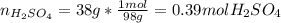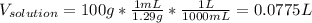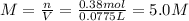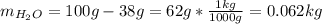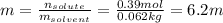Chemistry, 05.06.2020 08:59 msjsnell29
Automobile battery acid is 38% H2SO4 and has a destiny of 1.29g/ml. Calculate the molality and the molarity of this solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

You know the right answer?
Automobile battery acid is 38% H2SO4 and has a destiny of 1.29g/ml. Calculate the molality and the m...
Questions




Computers and Technology, 13.08.2021 04:20

Mathematics, 13.08.2021 04:20




Social Studies, 13.08.2021 04:20

Mathematics, 13.08.2021 04:20


Mathematics, 13.08.2021 04:20

Mathematics, 13.08.2021 04:20






Chemistry, 13.08.2021 04:20

Mathematics, 13.08.2021 04:20