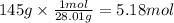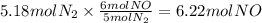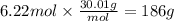Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
How many grams of NO are required to produce 145 g of N2 in the following reaction?
4NH3(g) + 6NO(g...
Questions




History, 27.01.2021 04:50

Biology, 27.01.2021 04:50

Mathematics, 27.01.2021 04:50

Mathematics, 27.01.2021 04:50

Mathematics, 27.01.2021 04:50

Mathematics, 27.01.2021 04:50


Mathematics, 27.01.2021 04:50



Mathematics, 27.01.2021 04:50

History, 27.01.2021 04:50


Mathematics, 27.01.2021 04:50



History, 27.01.2021 04:50