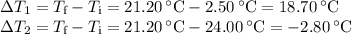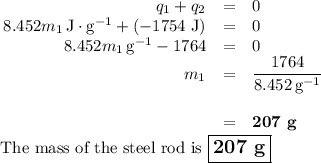CHEM EXPERT NEEDED***
A volume of 150.0 mL of H2O is initially at 24.00 °C. A chilled steel rod at 2.50 °C is placed in the water and the final temperature of the system is 21.20 °C.
Specific heat of water = 4.184 J/(g⋅∘C) and the specific heat of steel = 0.452 J/(g⋅∘C)
Write the equation and calculate the mass of the rod.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
CHEM EXPERT NEEDED***
A volume of 150.0 mL of H2O is initially at 24.00 °C. A chilled steel rod at...
Questions




Physics, 28.06.2019 03:00

Mathematics, 28.06.2019 03:00

Geography, 28.06.2019 03:00

Mathematics, 28.06.2019 03:00


Mathematics, 28.06.2019 03:00


History, 28.06.2019 03:00

Mathematics, 28.06.2019 03:00




English, 28.06.2019 03:00