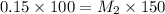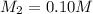Chemistry, 04.06.2020 17:58 swkgp3cevk
If I add water to 100mL of a 0.15 M NaOH solution until the final volume is 150mL, what will the molarity of the diluted solution be?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

You know the right answer?
If I add water to 100mL of a 0.15 M NaOH solution until the final volume is 150mL, what will the mol...
Questions








Computers and Technology, 06.05.2020 06:14

Mathematics, 06.05.2020 06:14

Mathematics, 06.05.2020 06:14


English, 06.05.2020 06:14

Mathematics, 06.05.2020 06:14




Biology, 06.05.2020 06:14

Mathematics, 06.05.2020 06:14

Mathematics, 06.05.2020 06:14

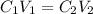
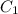 = concentration of concentrated solution = 0.15 M
= concentration of concentrated solution = 0.15 M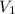 = volume of concentrated solution = 100 ml
= volume of concentrated solution = 100 ml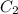 = concentration of diluted solution= ?
= concentration of diluted solution= ?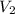 = volume of diluted solution= 150 ml
= volume of diluted solution= 150 ml