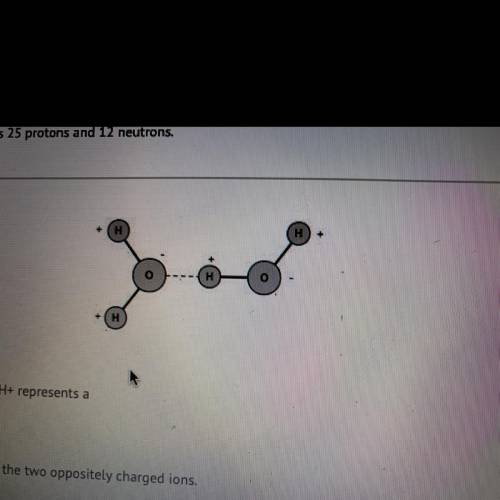
The dotted line between 0- and H+ represents a
A)
covalent bond between the two opposite...

The dotted line between 0- and H+ represents a
A)
covalent bond between the two oppositely charged ions.
B)
hydrogen bond, a weak bond between a hydrogen and an electronegative
atom.
C)
strong, short-distance bond that is responsible for the unique properties of
water.
polar covalent bond that results from the unequal and opposite charges of
the ions.
D)

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
Questions


Mathematics, 14.05.2021 18:10


Physics, 14.05.2021 18:10



Social Studies, 14.05.2021 18:10

Mathematics, 14.05.2021 18:10


Physics, 14.05.2021 18:10



Mathematics, 14.05.2021 18:10


History, 14.05.2021 18:10



Mathematics, 14.05.2021 18:10

Mathematics, 14.05.2021 18:10

History, 14.05.2021 18:10



