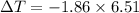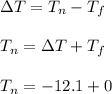What is the freezing point of a solution of 210.0 g of glycerol, formula C3H8O3, dissolved in 350. g of water? Careful. First get molar mass and use molar mass to determine molality concentration. Then use freeze pt. depression formula to determine the change in freezing pt. Then determine the new freeze point. The freezing point depression constant for water is Kf= -1.86 oCelcius/molal. Report your answer rounded to 1 decimal point and do not include units.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1
You know the right answer?
What is the freezing point of a solution of 210.0 g of glycerol, formula C3H8O3, dissolved in 350. g...
Questions

Mathematics, 12.11.2020 22:50



Chemistry, 12.11.2020 22:50


Mathematics, 12.11.2020 22:50


Mathematics, 12.11.2020 22:50


Computers and Technology, 12.11.2020 22:50


Chemistry, 12.11.2020 22:50





History, 12.11.2020 22:50



Mathematics, 12.11.2020 22:50

 Freezing point depression constant for water, Kf = 0.512 °C/m
Freezing point depression constant for water, Kf = 0.512 °C/m
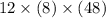
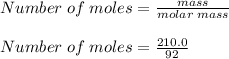
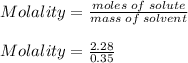
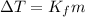
 is the change in temperature.
Kf is the molal freezing point constant.
m is the molality of solution.
is the change in temperature.
Kf is the molal freezing point constant.
m is the molality of solution.