
Chemistry, 01.06.2020 17:59 lilchannelll4125
Experiments have shown that the equilibrium constant Kp for the reaction NO(g) + NO2(g) ⇌ N2O3(g) is 0.03. A chamber with a constant total pressure initially contains NO with a partial pressure of 200 MPa, NO2 with a partial pressure of 250 MPa, and N2O3 with a partial pressure of 50 MPa. What will occur over time?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1


Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

You know the right answer?
Experiments have shown that the equilibrium constant Kp for the reaction NO(g) + NO2(g) ⇌ N2O3(g) is...
Questions



Mathematics, 18.09.2021 19:00



Mathematics, 18.09.2021 19:00

Physics, 18.09.2021 19:00

Mathematics, 18.09.2021 19:00

Biology, 18.09.2021 19:00




Mathematics, 18.09.2021 19:10


Mathematics, 18.09.2021 19:10

Mathematics, 18.09.2021 19:10


Mathematics, 18.09.2021 19:10


English, 18.09.2021 19:10

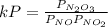 = 0.03
= 0.03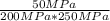 = 0.001
= 0.001

