
Chemistry, 31.05.2020 03:00 cookiemonster0476
Hard water often contains dissolved Ca2+ and Mg2+ ions. One way to soften water is to add phosphates. The phosphate ion forms insoluble precipitates with calcium and magnesium ions, removing them from solution. Suppose that a solution is 0.054 M in calcium chloride and 0.093 M in magnesium nitrate. What mass of sodium phosphate would have to be added to 1.4 L of this solution to completely eliminate the hard water ions? Assume complete reaction. Enter a numerical answer only, in terms of grams to two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2


Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Hard water often contains dissolved Ca2+ and Mg2+ ions. One way to soften water is to add phosphates...
Questions

Mathematics, 06.04.2020 19:00

Mathematics, 06.04.2020 19:00



Mathematics, 06.04.2020 19:00


Mathematics, 06.04.2020 19:00



English, 06.04.2020 19:00

History, 06.04.2020 19:00

Computers and Technology, 06.04.2020 19:00

Social Studies, 06.04.2020 19:00


Spanish, 06.04.2020 19:00

Mathematics, 06.04.2020 19:00


Business, 06.04.2020 19:00


Mathematics, 06.04.2020 19:00

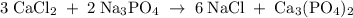
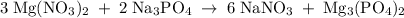
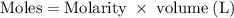
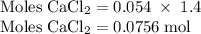
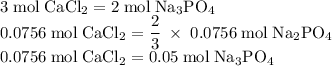
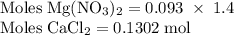
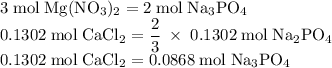
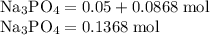
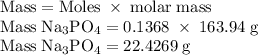
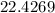 grams of sodium phosphate must be added to 1.4 L of this solution to completely eliminate the hard water ions
grams of sodium phosphate must be added to 1.4 L of this solution to completely eliminate the hard water ions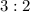
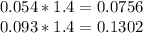
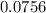 mole of CaCl2 is equal to
mole of CaCl2 is equal to 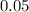 Na3PO4
Na3PO4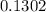 mole of CaCl2 is equal to
mole of CaCl2 is equal to 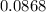 Na3PO4
Na3PO4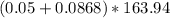 g/mol
g/mol 

