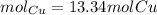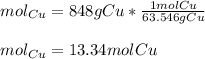Chemistry, 31.05.2020 00:03 spetgrave069
A sample of copper metal has a mass of 848 grams. How many moles of copper are in the sample? 13.34 mol Cu, 13.3 mol Cu, 12.9 mol Cu

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
A sample of copper metal has a mass of 848 grams. How many moles of copper are in the sample? 13.34...
Questions

Mathematics, 28.04.2021 01:00




History, 28.04.2021 01:00

Chemistry, 28.04.2021 01:00

Chemistry, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00

English, 28.04.2021 01:00




Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00

English, 28.04.2021 01:00



Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00