
Chemistry, 30.05.2020 06:00 caldonia2018
Balance the equation NH3+O2--->N2+H2O given 1.37 mol of the reactant NH3 , determine the corresponding amount of O2. Answer in units of mol.?
Find the corresponding amount of N2. Answer in units of mol.
Find the corresponding amount of H2O. Answer in units of mol.
Given 3.82 mol of the product N2, find the corresponding amount of NH3. Answer in units of mol.
Find the corresponding amount of O2. Answer in units of mol.
Find the corresponding amount of N2. Answer in units of mol.
Find the corresponding amount of H2O. Answer in units of mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Balance the equation NH3+O2--->N2+H2O given 1.37 mol of the reactant NH3 , determine the correspo...
Questions

English, 11.10.2020 19:01


Mathematics, 11.10.2020 19:01

Mathematics, 11.10.2020 19:01


Advanced Placement (AP), 11.10.2020 19:01

Mathematics, 11.10.2020 19:01

Mathematics, 11.10.2020 19:01

Mathematics, 11.10.2020 19:01


Biology, 11.10.2020 19:01



Mathematics, 11.10.2020 19:01


Mathematics, 11.10.2020 19:01

Mathematics, 11.10.2020 19:01


History, 11.10.2020 19:01

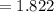 moles
moles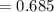 moles
moles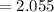 moles
moles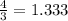 moles of Oxygems
moles of Oxygems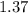 moles of NH3 shall combine with
moles of NH3 shall combine with 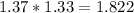 moles of oxygen (O2)
moles of oxygen (O2)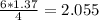 moles of water
moles of water

