
Chemistry, 29.05.2020 22:58 yulimariu27
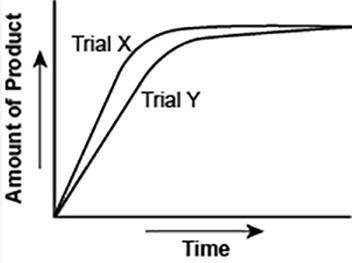
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A) Trial X, because the final volume of product formed is lower than Trial Y.
B) Trial X, because this reaction was initially fast and later stopped completely.
C) Trial Y, because the reaction was initially slow and later stopped completely.
D) Trial Y, because the volume of product formed per unit time is lower than Trial X.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions

Mathematics, 29.03.2021 21:20

English, 29.03.2021 21:20


Computers and Technology, 29.03.2021 21:20



Computers and Technology, 29.03.2021 21:20



Arts, 29.03.2021 21:20


Mathematics, 29.03.2021 21:20


Biology, 29.03.2021 21:20

Mathematics, 29.03.2021 21:20

Physics, 29.03.2021 21:20

Physics, 29.03.2021 21:20

Spanish, 29.03.2021 21:20

Mathematics, 29.03.2021 21:20

Mathematics, 29.03.2021 21:20



