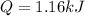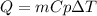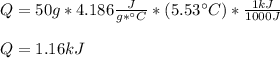Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
How much heat, in kJ, is required to raise the temperature of 50 g of water by 5.53°C? (Round to the...
Questions

Physics, 17.09.2019 18:00

Chemistry, 17.09.2019 18:00




Mathematics, 17.09.2019 18:00




Mathematics, 17.09.2019 18:00

Biology, 17.09.2019 18:00



Mathematics, 17.09.2019 18:00

English, 17.09.2019 18:00

Social Studies, 17.09.2019 18:00

Mathematics, 17.09.2019 18:00


History, 17.09.2019 18:00

Mathematics, 17.09.2019 18:00