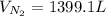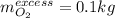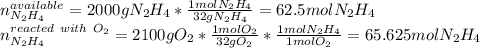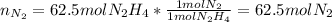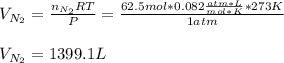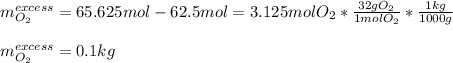Chemistry, 29.05.2020 22:57 bvargas786p7aa8y
Hydrazine (N2H4) is used as rocket fuel. It reacts with oxygen to form nitrogen and water.
N2H4(l) + O2(g) N2(g) + 2H2O(g)
a. How many liters of N2 (at STP) form when 2.0 kg N2H4 reacts with 2.1 kg O2?
b. How many grams of the excess reagent remain after the reaction?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
Hydrazine (N2H4) is used as rocket fuel. It reacts with oxygen to form nitrogen and water.
Questions

English, 03.12.2020 19:20

Mathematics, 03.12.2020 19:20

Mathematics, 03.12.2020 19:20

Mathematics, 03.12.2020 19:20

Chemistry, 03.12.2020 19:20



Mathematics, 03.12.2020 19:20

Chemistry, 03.12.2020 19:20

History, 03.12.2020 19:20

Mathematics, 03.12.2020 19:20

History, 03.12.2020 19:20

Social Studies, 03.12.2020 19:20


German, 03.12.2020 19:20


Mathematics, 03.12.2020 19:20