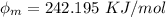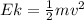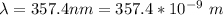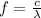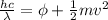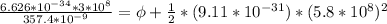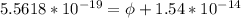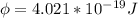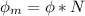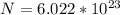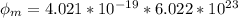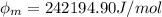Chemistry, 29.05.2020 09:57 lilyrockstarmag
In order to comply with the requirement that energy be conserved, Einstein showed in the photoelectric effect that the energy of a photon (h) absorbed by a metal is the sum of the work function (), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron: h = + Ek. When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is (in kJ/mol) of potassium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
In order to comply with the requirement that energy be conserved, Einstein showed in the photoelectr...
Questions

Mathematics, 18.03.2021 02:30

Social Studies, 18.03.2021 02:30

History, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30



History, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30

Biology, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30


History, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30


Chemistry, 18.03.2021 02:30

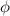 ), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron:
), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron: 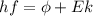 . When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is work function (in kJ/mol) of potassium?
. When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is work function (in kJ/mol) of potassium?