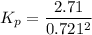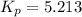For the reaction: 2 A (g) + B (s)= 2 C(s) + D (g)
At 298 K in a 10.0 L vessel, the equilibrium...

Chemistry, 29.05.2020 19:03 amberskids2
For the reaction: 2 A (g) + B (s)= 2 C(s) + D (g)
At 298 K in a 10.0 L vessel, the equilibrium values are as follows: 0.721 atm of A, 4.18 mol of B, 6.25 mol of C, and 2.71 atm of D. What is the value of the equilibrium constant?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Questions



Health, 30.09.2019 06:00

Social Studies, 30.09.2019 06:00

Social Studies, 30.09.2019 06:00

Mathematics, 30.09.2019 06:00


Health, 30.09.2019 06:00



History, 30.09.2019 06:00


Business, 30.09.2019 06:00

Social Studies, 30.09.2019 06:00


History, 30.09.2019 06:00



Spanish, 30.09.2019 06:00

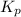 (equilibrium constant) is referred to as the partial pressure of product divided by the partial pressure of reactant with each pressure term raised to power that is equal to its stoichiometric coefficient in balanced equation
.
(equilibrium constant) is referred to as the partial pressure of product divided by the partial pressure of reactant with each pressure term raised to power that is equal to its stoichiometric coefficient in balanced equation
.![K_p = \dfrac{[D]}{[A]^2}](/tpl/images/0670/4630/dcbe2.png)