
Chemistry, 29.05.2020 06:02 angelZ3947
The first two steps in the industrial synthesis of nitric acid produce nitrogen dioxide from ammonia: The net reaction is: Write an equation that gives the overall equilibrium constant in terms of the equilibrium constants and . If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2
You know the right answer?
The first two steps in the industrial synthesis of nitric acid produce nitrogen dioxide from ammonia...
Questions


Computers and Technology, 13.02.2020 18:36




English, 13.02.2020 18:36


History, 13.02.2020 18:36


Mathematics, 13.02.2020 18:36



History, 13.02.2020 18:36

Mathematics, 13.02.2020 18:36

Biology, 13.02.2020 18:36




Computers and Technology, 13.02.2020 18:36

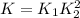
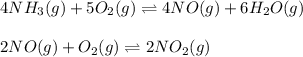
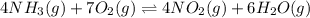
![K_1=\frac{[NO]^4[H_2O]^6}{[NH_3]^4[O_2]^5}\\ \\K_2=\frac{[NO_2]^2}{[NO]^2[O_2]}](/tpl/images/0669/9771/004ed.png)
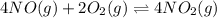
![K_2^{new}=\frac{[NO_2]^4}{[NO]^4[O_2]^2}](/tpl/images/0669/9771/b0ba1.png)
![K_1*K_2^2=\frac{[NO]^4[H_2O]^6}{[NH_3]^4[O_2]^5}*\frac{[NO_2]^4}{[NO]^4[O_2]^2}=\frac{[H_2O]^6[NO_2]^4}{[NH_3]^4[O_2]^7}](/tpl/images/0669/9771/5162f.png)
![K=\frac{[H_2O]^6[NO_2]^4}{[NH_3]^4[O_2]^7}](/tpl/images/0669/9771/dc773.png)


