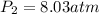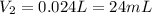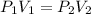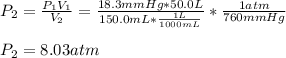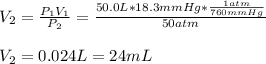Chemistry, 29.05.2020 03:02 Franklyn3834
3. A 50.0 L sample of gas collected in the upper atmosphere at a pressure of 18.3 mmHg is compressed into a 150.0 mL container at the same temperature.
a. What is the new pressure, in atm?
b. To what volume would the original sample have had to be compressed to exert a pressure of 10.0 atm?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
3. A 50.0 L sample of gas collected in the upper atmosphere at a pressure of 18.3 mmHg is compressed...
Questions

Mathematics, 28.07.2019 20:00

Chemistry, 28.07.2019 20:00




Mathematics, 28.07.2019 20:00

Geography, 28.07.2019 20:00

Biology, 28.07.2019 20:00

Mathematics, 28.07.2019 20:00

Mathematics, 28.07.2019 20:00

Health, 28.07.2019 20:00


Mathematics, 28.07.2019 20:00




English, 28.07.2019 20:00

Mathematics, 28.07.2019 20:00

Mathematics, 28.07.2019 20:00

Mathematics, 28.07.2019 20:00