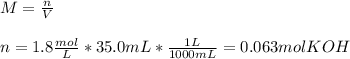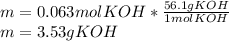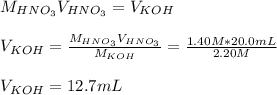Chemistry, 27.05.2020 23:01 sneakersolequeen
PLEASE SHOW WORK
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the concentration of the acetic acid were 2.40 M, what would be the concentration of H + at equilibrium?
2) You have a solution that is 1.75 M HCN. If the K a is 9.9 × 10 –8 , calculate the pH of the solution.
3) How many grams of KOH are needed to neutralize 35.0 mL of 1.8 M HCl?
4) How many mL of 2.20 M KOH are needed to neutralize 20.0 mL of 1.40 M HNO 3 ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1
You know the right answer?
PLEASE SHOW WORK
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the...
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the...
Questions




Mathematics, 15.10.2019 15:50

Business, 15.10.2019 15:50

History, 15.10.2019 15:50

Geography, 15.10.2019 15:50



Computers and Technology, 15.10.2019 15:50

History, 15.10.2019 15:50

Biology, 15.10.2019 15:50

English, 15.10.2019 15:50


Computers and Technology, 15.10.2019 15:50

History, 15.10.2019 15:50

Mathematics, 15.10.2019 15:50


English, 15.10.2019 15:50

![[H^+]_{eq}=0.00029M](/tpl/images/0667/7635/6d441.png)
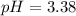
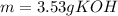
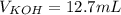
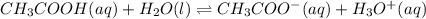
![Ka=\frac{[CH_3COO^-][H_3O^+]}{[CH_3COOH]}](/tpl/images/0667/7635/3cdd5.png)
 due to reaction's extent we have:
due to reaction's extent we have: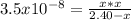
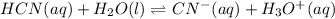
![K=\frac{[CN^-][H_3O^+]}{[HCN]}](/tpl/images/0667/7635/eab5d.png)
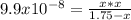
![[H^+]_{eq}=0.000416 M](/tpl/images/0667/7635/3b132.png)
![pH=-log([H^+]_{eq})=-log(0.000416)\\\\pH=3.38](/tpl/images/0667/7635/c9e0e.png)