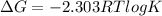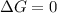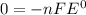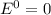Given the chemical reaction: Hg2+(aq) + Cu(s) + Hg(s) + Cu2+(aq)
When the reaction reaches equ...

Chemistry, 28.05.2020 22:04 wolfgirl2431
Given the chemical reaction: Hg2+(aq) + Cu(s) + Hg(s) + Cu2+(aq)
When the reaction reaches equilibrium, the cell potential will be
1
-0.51 V
2.
0.00 V
3.
0.51 V
4.
1.19 V

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Questions




Mathematics, 20.10.2020 20:01


History, 20.10.2020 20:01

Physics, 20.10.2020 20:01




Advanced Placement (AP), 20.10.2020 20:01

Engineering, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

English, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01




English, 20.10.2020 20:01

Biology, 20.10.2020 20:01