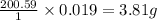Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
What is the mass, in grams, of a sample of 1.20 × 1022 atoms of mercury (hg)? show your work or exp...
Questions

Mathematics, 11.03.2021 05:40


Advanced Placement (AP), 11.03.2021 05:40

Health, 11.03.2021 05:40

Mathematics, 11.03.2021 05:40

Mathematics, 11.03.2021 05:40

Mathematics, 11.03.2021 05:40



Mathematics, 11.03.2021 05:40

English, 11.03.2021 05:40

Social Studies, 11.03.2021 05:40

Computers and Technology, 11.03.2021 05:40





English, 11.03.2021 05:40


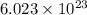 of particles.
of particles.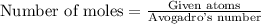
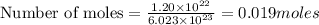 (1L=1000ml)
(1L=1000ml)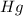 atom weighs = 200.59 g
atom weighs = 200.59 g