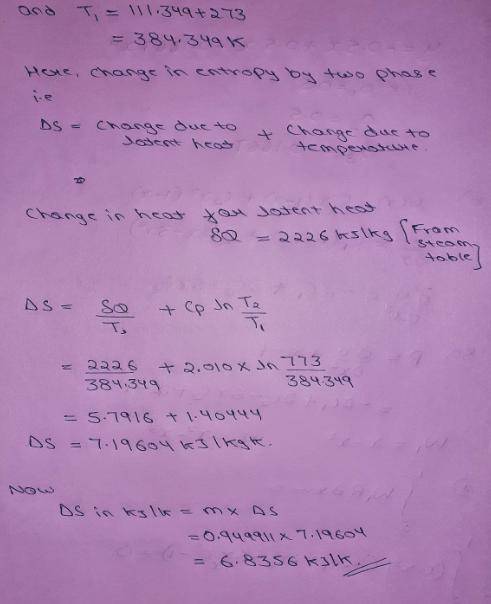Chemistry, 28.05.2020 19:01 donaldplawlerp5cctt
An insulated piston-cylinder device contains 1 L of saturated liquid water at pressure of 150 kPa. An electric resistance heater inside the cylinder is now turned on, and 2869.47 kJ of heat is transferred to the water. The inside H2O pressure maintains constant at 150 kPa during the process. Determine: the entropy change of the water during this heating process, in kJ/K.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3


Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
An insulated piston-cylinder device contains 1 L of saturated liquid water at pressure of 150 kPa. A...
Questions

Social Studies, 26.08.2019 00:10

Chemistry, 26.08.2019 00:10

Mathematics, 26.08.2019 00:10

Mathematics, 26.08.2019 00:10


Mathematics, 26.08.2019 00:10



Chemistry, 26.08.2019 00:10



Mathematics, 26.08.2019 00:10


Health, 26.08.2019 00:20


Physics, 26.08.2019 00:20


English, 26.08.2019 00:20

History, 26.08.2019 00:20