
Chemistry, 28.05.2020 17:58 sofiaarmy12
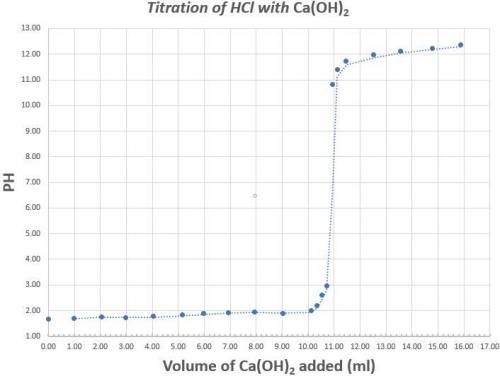
Read the graph to the nearest tenth for both pH and volume of calcium hydroxide. 25.0ml of a 1.70x10-4M solution of Hydrochloric acid was titrated with Calcium hydroxide. The above graph was generated when the Hydrochloric acid was titrated with Calcium hydroxide. Determine the concentration (in M) of the Calcium hydroxide. What is the coefficient of the scientific notation answer for the concentration of Calcium Hydroxide.
Determine the percent error if the known concentration of calcium hydroxide is 6.30x10-4M. (Do not put your answer in scientific notation).

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
Read the graph to the nearest tenth for both pH and volume of calcium hydroxide. 25.0ml of a 1.70x10...
Questions





Mathematics, 06.05.2020 06:07





Chemistry, 06.05.2020 06:07




History, 06.05.2020 06:07

English, 06.05.2020 06:07



English, 06.05.2020 06:07




