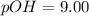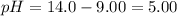2. Classify the following solutions as acidic, basic, or neutral at 25OC.
a. pH = 13.00
...

Chemistry, 28.05.2020 09:58 alexandrarosete7
2. Classify the following solutions as acidic, basic, or neutral at 25OC.
a. pH = 13.00
b. [H3O+] = 1.0 x 10-12 M
c. pOH = 5.00
d. [OH-]= 1.0 x 10-9M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Questions


English, 18.08.2019 16:30


Health, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30


Chemistry, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30

English, 18.08.2019 16:30

Geography, 18.08.2019 16:30


Mathematics, 18.08.2019 16:30

German, 18.08.2019 16:30




English, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30

History, 18.08.2019 16:30

![[H_3O^+]=1.0\times 10^{-12}](/tpl/images/0668/5415/5039a.png) : basic
: basic ![[OH^-]=1.0\times 10^{-9}](/tpl/images/0668/5415/579ee.png) : acidic
: acidic![pH=-\log [H_3O^+]](/tpl/images/0668/5415/841e8.png)
![pH=-\log[1.0\times 10^{-12}]](/tpl/images/0668/5415/b3c7d.png)
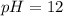
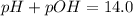

![pOH=-\log[1.0\times 10^{-9}]](/tpl/images/0668/5415/f1089.png)