
Chemistry, 27.05.2020 19:01 laneycasey9058
One liter of oxygen gas at standard temperature and pressure has a mass of 1.43 g. The same volume of hydrogen gas under these conditions is 0.089 g. If both volumes contain the same number of gas particles (according to Avogadro's hypothesis), how can this difference in mass be explained?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
One liter of oxygen gas at standard temperature and pressure has a mass of 1.43 g. The same volume o...
Questions



History, 20.01.2020 11:31

English, 20.01.2020 11:31

Physics, 20.01.2020 11:31

Arts, 20.01.2020 11:31

Mathematics, 20.01.2020 11:31

Chemistry, 20.01.2020 11:31

Mathematics, 20.01.2020 11:31

Mathematics, 20.01.2020 11:31

Biology, 20.01.2020 11:31

Physics, 20.01.2020 11:31


Biology, 20.01.2020 12:31



Health, 20.01.2020 12:31



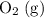 is larger than that of
is larger than that of 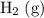 (by a factor of about
(by a factor of about 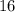 .) Therefore, the mass of the
.) Therefore, the mass of the 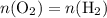 .
.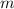 is different from the number of gas particles
is different from the number of gas particles 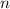 in it. In particular, if all particles in this gas have a molar mass of
in it. In particular, if all particles in this gas have a molar mass of 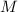 , then:
, then: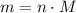 .
.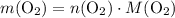 .
.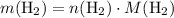 .
.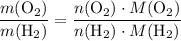 .
.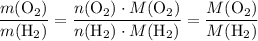 .
. :
: 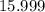 .
.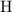 :
: 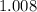 .
.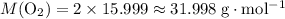 .
.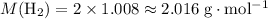 .
.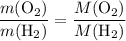 :
: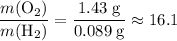 .Right-hand side:
.Right-hand side: 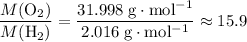 .
.

