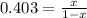Chemistry, 28.05.2020 04:57 jacquetpaul1969
A key step in the extraction of iron from its ore is FeO(s) + CO(g) ⇋ Fe(s) + CO2(g) Kp =0.403 at 1000°C. What are the equilibrium partial pressures of carbon monoxide and carbon dioxide when 1.00 atm of carbon monoxide and excess iron(II) oxide react in a sealed container at 1000°C. write Kp expression, do ICE chart with pressures, 0.287 atm CO2 and 0.713 atm CO

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
A key step in the extraction of iron from its ore is FeO(s) + CO(g) ⇋ Fe(s) + CO2(g) Kp =0.403 at 10...
Questions

Mathematics, 22.01.2020 07:31

Mathematics, 22.01.2020 07:31


English, 22.01.2020 07:31

Physics, 22.01.2020 07:31

History, 22.01.2020 07:31

Advanced Placement (AP), 22.01.2020 07:31

Mathematics, 22.01.2020 07:31





Mathematics, 22.01.2020 07:31

Biology, 22.01.2020 07:31




Mathematics, 22.01.2020 07:31

Mathematics, 22.01.2020 07:31

Mathematics, 22.01.2020 07:31

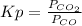 = 0.403
= 0.403