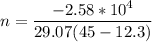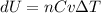Nitrogen gas in an expandable container is cooled from 45.0 C to 12.3 C with the pressure held constant at 2.58 ∗ 105 Pa. The total heat liberated by the gas is 2.58 ∗ 104 J. Assume that the gas may be treated as ideal. Find (a) the number of moles of gas; (b) the change in internal energy of the gas; (c) the work done by the gas. (d) How much heat woul

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Nitrogen gas in an expandable container is cooled from 45.0 C to 12.3 C with the pressure held const...
Questions

Mathematics, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40


Mathematics, 03.03.2021 21:40

History, 03.03.2021 21:40



History, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40




Biology, 03.03.2021 21:40

Social Studies, 03.03.2021 21:40

Physics, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40

French, 03.03.2021 21:40


Mathematics, 03.03.2021 21:40

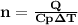
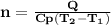
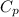 is the specific heat at constant pressure of Nitrogen gas which is = 29.07 J/mol/K
is the specific heat at constant pressure of Nitrogen gas which is = 29.07 J/mol/K