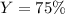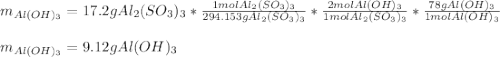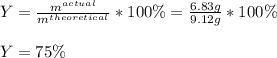Chemistry, 28.05.2020 02:06 YoVeoAnime
If you start with 17.2 g of Al2(SO3)3 and you produce 6.83 gAl(OH)3, what is your percent yield for this reaction?
Al2(SO3)3 + 6 NaOH → 3 Na2SO3 + 2 Al(OH)3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
If you start with 17.2 g of Al2(SO3)3 and you produce 6.83 gAl(OH)3, what is your percent yield for...
Questions



Social Studies, 07.08.2019 08:10


Mathematics, 07.08.2019 09:10


Mathematics, 07.08.2019 09:10

English, 07.08.2019 09:10


Chemistry, 07.08.2019 09:10




Mathematics, 07.08.2019 09:10



Mathematics, 07.08.2019 09:10