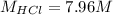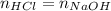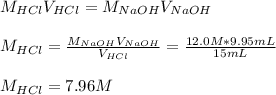Chemistry, 28.05.2020 01:06 psychocatgirl1
Concentrated hydrochloric acid is a solution that is 37.5% mass per unit volume HCl(aq) in water. An old bottle of HCl has an unknown concentration. What is the concentration of hydrochloric acid, [HCl], in the old bottle, if 9.95 mL of 12.0 M NaOH(aq) is required to reach the equivalence point when added to 15 mL of acid?What is the concentration of HCl(aq)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2
You know the right answer?
Concentrated hydrochloric acid is a solution that is 37.5% mass per unit volume HCl(aq) in water. An...
Questions

English, 28.01.2021 20:50

Mathematics, 28.01.2021 20:50

Chemistry, 28.01.2021 20:50

History, 28.01.2021 20:50

Mathematics, 28.01.2021 20:50

Computers and Technology, 28.01.2021 20:50


English, 28.01.2021 20:50

Mathematics, 28.01.2021 20:50




Mathematics, 28.01.2021 20:50

Biology, 28.01.2021 20:50

Advanced Placement (AP), 28.01.2021 20:50




Biology, 28.01.2021 20:50

Mathematics, 28.01.2021 20:50