5. A standardized mass was known to weigh exactly 5.0000g. It was weighed on a newly
acquired,...

Chemistry, 28.05.2020 01:01 floreschachi2873
5. A standardized mass was known to weigh exactly 5.0000g. It was weighed on a newly
acquired, uncalibrated balance as 5.001g. Calculate the absolute error and the percent error.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1

Chemistry, 23.06.2019 11:40
An electron moved from a lower energy level to a higher energy level. what most likely happened during the transition? a random amount of light was released. a fixed amount of energy was absorbed. a fixed amount of energy was released. a random amount of light was absorbed.
Answers: 1
You know the right answer?
Questions



History, 04.02.2021 01:00

History, 04.02.2021 01:00



History, 04.02.2021 01:00

Biology, 04.02.2021 01:00

Mathematics, 04.02.2021 01:00


Mathematics, 04.02.2021 01:00



Social Studies, 04.02.2021 01:00

Biology, 04.02.2021 01:00


Mathematics, 04.02.2021 01:00


Biology, 04.02.2021 01:00

Mathematics, 04.02.2021 01:00

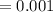
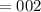 5
5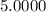 grams
grams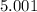 grams
grams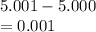
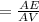
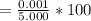
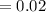 %
%

