
Chemistry, 27.05.2020 04:58 phsycotic121
What is the concentration of the silver ion in silver chromate, Ag₂CrO₄, if its solubility product constant (Kₛₚ) is 1.2 x 10⁻¹². Hint: write the equation first! *
2 points
1.4 x 10⁻⁵
1.1 x 10⁻⁹
1.6 x 10⁻¹²
2.4 x 10⁻¹²

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1


Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

You know the right answer?
What is the concentration of the silver ion in silver chromate, Ag₂CrO₄, if its solubility product c...
Questions

Mathematics, 29.12.2019 05:31



English, 29.12.2019 05:31


Biology, 29.12.2019 05:31

Mathematics, 29.12.2019 05:31

Mathematics, 29.12.2019 05:31

Biology, 29.12.2019 05:31


Mathematics, 29.12.2019 05:31

Chemistry, 29.12.2019 05:31

Mathematics, 29.12.2019 05:31


Mathematics, 29.12.2019 05:31


Health, 29.12.2019 05:31

Mathematics, 29.12.2019 05:31


![[Ag^+]=1.3x10^{-4}M](/tpl/images/0666/7571/0a833.png)
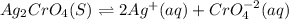
![Ksp=[Ag]^2[CrO_4^{-2}]](/tpl/images/0666/7571/75fed.png)
 due to the dissolution of silver chromate, we obtain:
due to the dissolution of silver chromate, we obtain: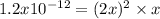
![x=\sqrt[3]{\frac{1.2x10^{-12}}{2^2} } = 6.7x10^{-5}M](/tpl/images/0666/7571/1e8f3.png)
![[Ag^+]=2x=2*6.7x10^{-5}M=1.3x10^{-4}M](/tpl/images/0666/7571/1be44.png)


