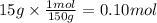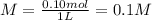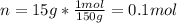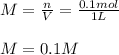Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
When light strikes a plane mirror, images form in locations where light does not actually reach. it only appears to the observer as though the light were coming from this position. what type of image is formed?
Answers: 2

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

You know the right answer?
You have 15g of sodium iodide (Nal), which has a molecular weight of 150 g/mol. You place it all in...
Questions



Chemistry, 03.04.2021 15:30

Physics, 03.04.2021 15:30

Mathematics, 03.04.2021 15:30


Mathematics, 03.04.2021 15:30

Mathematics, 03.04.2021 15:30

Mathematics, 03.04.2021 15:30




Geography, 03.04.2021 15:40






English, 03.04.2021 15:40