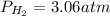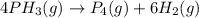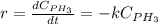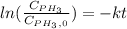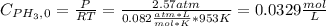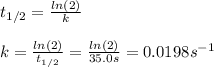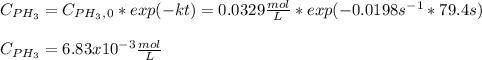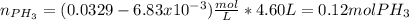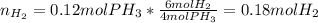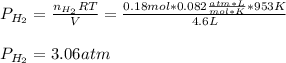Chemistry, 26.05.2020 18:57 eldiamonte
Phosphine, PH3PH3 , is a colorless, toxic gas that is used in the production of semiconductors as well as in the farming industry. When heated, phosphine decomposes into phosphorus and hydrogen gases. 4PH3(g)⟶P4(g)+6H2(g)4PH3(g)⟶P4(g)+6 H2(g) This decomposition is first order with respect to phosphine, and has a half‑life of 35.0 s at 953 K. Calculate the partial pressure of hydrogen gas that is present after 79.4 s79.4 s if a 4.60 L4.60 L vessel containing 2.57 atm2.57 atm of phosphine gas is heated to 953 K

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2


Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
Phosphine, PH3PH3 , is a colorless, toxic gas that is used in the production of semiconductors as we...
Questions

Mathematics, 14.07.2021 22:40

Social Studies, 14.07.2021 22:40


Social Studies, 14.07.2021 22:40

Mathematics, 14.07.2021 22:40




Mathematics, 14.07.2021 22:40

History, 14.07.2021 22:40


Advanced Placement (AP), 14.07.2021 22:40






Mathematics, 14.07.2021 22:50