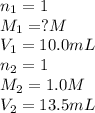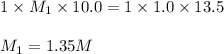Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
A 10.0 mL sample of HNO3 was exactly neutralized by 13.5 mL of 1.0 M KOH. What is the molarity of th...
Questions

History, 02.11.2019 14:31


Physics, 02.11.2019 14:31


History, 02.11.2019 14:31


History, 02.11.2019 14:31


Biology, 02.11.2019 14:31




History, 02.11.2019 14:31





Chemistry, 02.11.2019 14:31


Mathematics, 02.11.2019 14:31

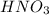 is 1.35 M
is 1.35 M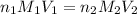
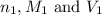 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 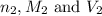 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.