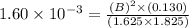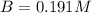At a certain temperature, the equilibrium constant for the chemical reaction shown is 1.60×10−3 . At equilibrium, the concentration of AB is 1.825 M, the concentration of BC is 1.625 M, and the concentration of AC is 0.130 M. Calculate the concentration of B at equilibrium. AB(aq)+BC(aq)↽−−⇀AC(aq)+2B(aq) AB ( aq ) + BC ( aq ) ↽ − − ⇀ AC ( aq ) + 2 B ( aq ) [B] =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 13:00
If volume remains the same while the mass of a substance the density of the substance
Answers: 1
You know the right answer?
At a certain temperature, the equilibrium constant for the chemical reaction shown is 1.60×10−3 . At...
Questions

Mathematics, 20.10.2019 10:50

Mathematics, 20.10.2019 10:50




Mathematics, 20.10.2019 10:50


History, 20.10.2019 10:50

Health, 20.10.2019 10:50


Social Studies, 20.10.2019 10:50

Biology, 20.10.2019 10:50

Spanish, 20.10.2019 10:50

Mathematics, 20.10.2019 10:50


Computers and Technology, 20.10.2019 10:50


Spanish, 20.10.2019 10:50

Mathematics, 20.10.2019 10:50

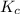
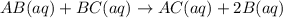
![K_c=\frac{[B]^2\times [AC]}{[BC]\times [AB]}](/tpl/images/0664/6603/29fb9.png)