Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2


Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
2. In the reaction H2(g) + b(0) + 2HI(), there are equilibrium partial pressures of PH, = 13.0 MPa,...
Questions




Biology, 15.06.2021 17:30

Mathematics, 15.06.2021 17:30


Mathematics, 15.06.2021 17:30

Mathematics, 15.06.2021 17:30




Business, 15.06.2021 17:30


English, 15.06.2021 17:30

Mathematics, 15.06.2021 17:30

Mathematics, 15.06.2021 17:30



Mathematics, 15.06.2021 17:30

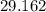
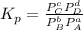
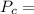
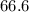 MPa and c is equal to two
MPa and c is equal to two MPa
MPa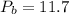 MPa
MPa