Chemistry, 23.05.2020 16:59 milkshakegrande101
A geochemist in the field takes a 34.0 mL sample of water from a rock pool lined with crystals of a certain mineral compound X. He notes the temperature of the pool, 22.0 °C, and caps the sample carefully. Back in the lab, the geochemist first dilutes the sample with distilled water to 750.0 mL. Then he filters it and evaporates all the water under vacuum. Crystals of X are left behind. The researcher washes, dries, and weighs the crystals. They weigh 0.31 g.
Using only the information above, can you calculate yes the solubility of X in the water at 17.0 °C? If yes, calculate it. Be sure your answer has a unit symbol and 3 significant digits.

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
A geochemist in the field takes a 34.0 mL sample of water from a rock pool lined with crystals of a...
Questions










Engineering, 26.08.2020 16:01




Business, 26.08.2020 16:01






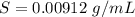
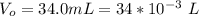
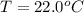
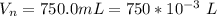
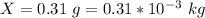
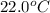 can be mathematically evaluate as
can be mathematically evaluate as