Chemistry, 22.05.2020 06:58 josephvcarter
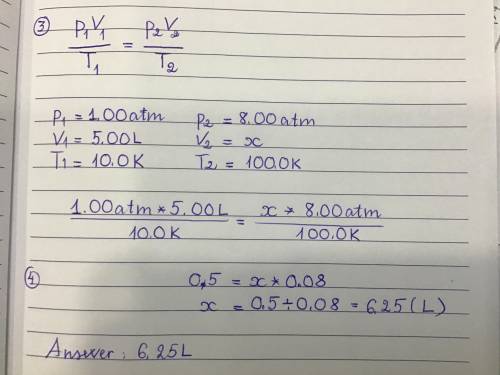
An ideal gas has a volume of 5.00L under a pressure of 1.00 atm and a temperature of 10.0 K. If the temperature is changed to 100.0 K while the pressure is increased to 8.00 atm what would be the new volume of the gas?
6.25L
1.60L
400.0L
16.0L

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
An ideal gas has a volume of 5.00L under a pressure of 1.00 atm and a temperature of 10.0 K. If the...
Questions



Chemistry, 01.07.2020 15:01



Mathematics, 01.07.2020 15:01


Computers and Technology, 01.07.2020 15:01

Biology, 01.07.2020 15:01





Spanish, 01.07.2020 15:01

History, 01.07.2020 15:01


History, 01.07.2020 15:01



Mathematics, 01.07.2020 15:01