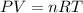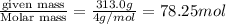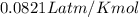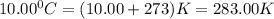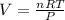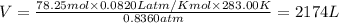Chemistry, 22.05.2020 07:59 harri26348
What must be the volume of a balloon that can hold 313.0 g of helium gas and ascend from sea level to an altitude, where the temperature is 10.00C and the pressure is 635.4 mmHg?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
What must be the volume of a balloon that can hold 313.0 g of helium gas and ascend from sea level t...
Questions


History, 26.09.2019 17:40


Social Studies, 26.09.2019 17:40







Mathematics, 26.09.2019 17:40



Mathematics, 26.09.2019 17:40






Social Studies, 26.09.2019 17:40