

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 13:20
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2
You know the right answer?
1. A solution was made by dissolving 0.837g of Ba(OH)2 in 100 mL of solution. What is the pOH and pH...
Questions


Mathematics, 17.11.2020 20:40


Mathematics, 17.11.2020 20:40


Mathematics, 17.11.2020 20:40


English, 17.11.2020 20:40



Physics, 17.11.2020 20:40

Mathematics, 17.11.2020 20:40

Physics, 17.11.2020 20:40


Advanced Placement (AP), 17.11.2020 20:40

Mathematics, 17.11.2020 20:40

Mathematics, 17.11.2020 20:40



![pH = 14 - pOH = 14 - (-log[OH^{-}]) = 14 + log[OH^{-}]](/tpl/images/0661/1440/d204c.png)
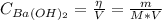
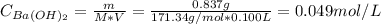
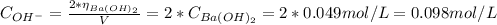
![pOH = -log[OH^{-}] = -log(0.098) = 1.01](/tpl/images/0661/1440/09f40.png)
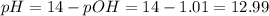
![\% I = \frac{[H^{+}]_{eq}}{[HA]_{0}}*100](/tpl/images/0661/1440/bf5f0.png)
![Ka = \frac{[CH_{3}COO^{-}]*[H_{3}O^{+}]}{[CH_{3}COOH]}](/tpl/images/0661/1440/7ec2b.png)
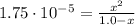
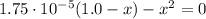 (1)
(1) ![\% I = \frac{[H^{+}]_{eq}}{[HA]_{0}}*100 = \frac{0.00417 M}{1.0 M}*100 = 0.417 \%](/tpl/images/0661/1440/a546b.png)
![pH = -log([H_{3}O^{+}]) = -log(0.00417) = 2.34](/tpl/images/0661/1440/23f0f.png)


