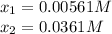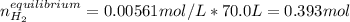Chemistry, 22.05.2020 02:10 Flameking1223
For the reaction
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calculate the number of moles of H2 that are present at equilibrium if a mixture of 0.680 mole of CO and 0.680 mole of H2O is heated to 700°C in a 70.0−L container.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

You know the right answer?
For the reaction
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calcu...
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calcu...
Questions

Mathematics, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40

Spanish, 05.11.2020 21:40


History, 05.11.2020 21:40

Biology, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40

Chemistry, 05.11.2020 21:40


Mathematics, 05.11.2020 21:40


Mathematics, 05.11.2020 21:40

Social Studies, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40

Social Studies, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40


Arts, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40

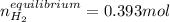
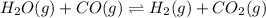
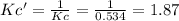
![Kc'=\frac{[H_2][CO_2]}{[H_2O][CO]}](/tpl/images/0660/7360/14ed3.png)
 due to the reaction extent:
due to the reaction extent:![Kc'=\frac{(x)(x)}{([H_2O]_0-x)([CO]_0-x)}=1.87](/tpl/images/0660/7360/2d81b.png)
![[H_2O]_0=[CO]_0=\frac{0.680mol}{70.0L}=0.0097M](/tpl/images/0660/7360/92f9f.png)