answer to the nearest whole number.

Chemistry, 21.05.2020 19:59 autumperry3599
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
CaCO3(s)– Cao(s) + CO2(g)
AGf. Cacoa = -1,128.76 kJ/mol
AGf, Cao = -604.17 kJ/mol
AGT, CO, = -394.4 kJ/mol
AGrx = what

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
answer to the nearest whole number.
Questions





English, 27.06.2020 01:01





Mathematics, 27.06.2020 01:01


Mathematics, 27.06.2020 01:01

Biology, 27.06.2020 01:01








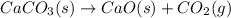
![\Delta G_{rxn}=\sum [n\times \Delta G_(product)]-\sum [n\times \Delta G_(reactant)]](/tpl/images/0659/5075/ad616.png)
![\Delta G_{rxn}=[(n_{CO_2}\times \Delta G_{CO_2})+(n_{CaO}\times \Delta G_{CaO})]-[(n_{CaCO_3}\times \Delta G_{CaCO_3})]](/tpl/images/0659/5075/3d6cf.png)
![\Delta G_{rxn}=[(1\times -394.4)+(1\times -604.17)]-[(1\times -1128.76)]](/tpl/images/0659/5075/691fa.png)


