
Chemistry, 21.05.2020 18:00 NathanaelLopez
Carbon disulfide is prepared by heating sulfur and charcoal. The chemical equation is ; =9.40 at 900 K How many grams of CS₂(g) can be prepared by heating 13.8 mol S₂(g) with excess carbon in a 8.60 L reaction vessel held at 900 K until equilibrium is attained?
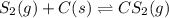

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
Carbon disulfide is prepared by heating sulfur and charcoal. The chemical equation is ; =9.40 at 900...
Questions

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01







Business, 10.09.2020 04:01




Mathematics, 10.09.2020 04:01


English, 10.09.2020 04:01




Health, 10.09.2020 04:01




