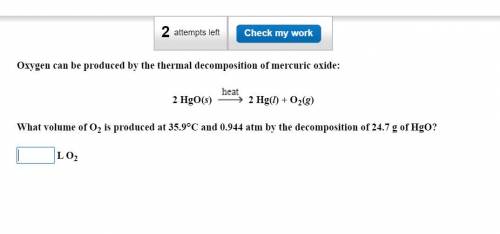
What volume of O2 is produced at 35.9°C and 0.944 atm by the decomposition of 24.7 g of HgO?
...

Chemistry, 21.05.2020 04:00 IsabelAyshi
What volume of O2 is produced at 35.9°C and 0.944 atm by the decomposition of 24.7 g of HgO?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
Questions





Mathematics, 03.07.2021 01:30

Mathematics, 03.07.2021 01:30



Business, 03.07.2021 01:30

Computers and Technology, 03.07.2021 01:30





Mathematics, 03.07.2021 01:30

Business, 03.07.2021 01:30






